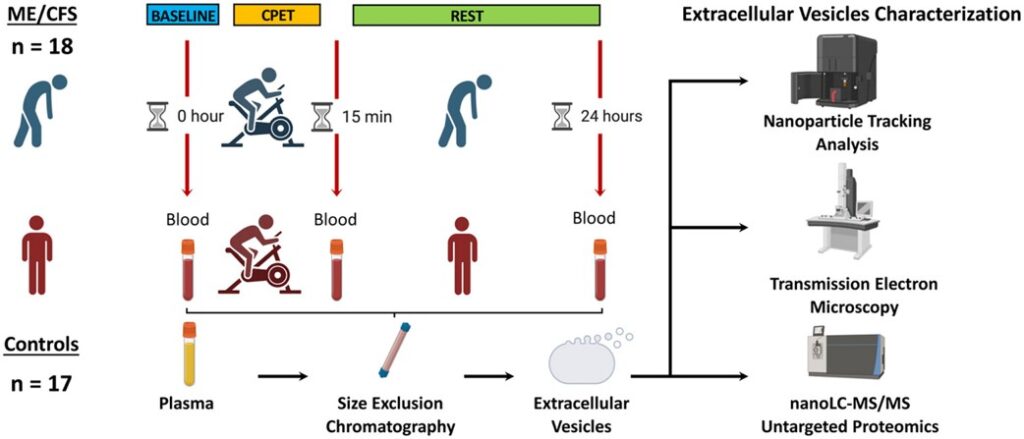
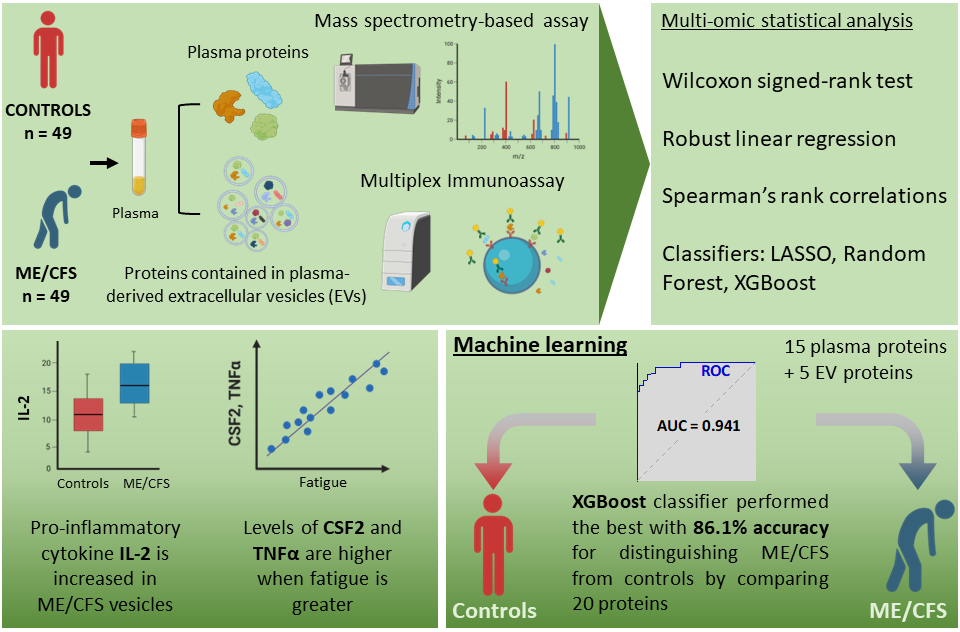
Out in the Journal of Translational Medicine, Betsy Keller et al. describes results from the Center’s large multi-site 2-day CPET study. The publication analyzes cardiopulmonary exercise testing (CPET) data collected over two days from 84 individuals with ME/CFS and 71 sedentary controls. Within this population, 55 sex, age, and fitness-matched pairs were also compared. This is the largest 2-day CPET study of ME/CFS to date.
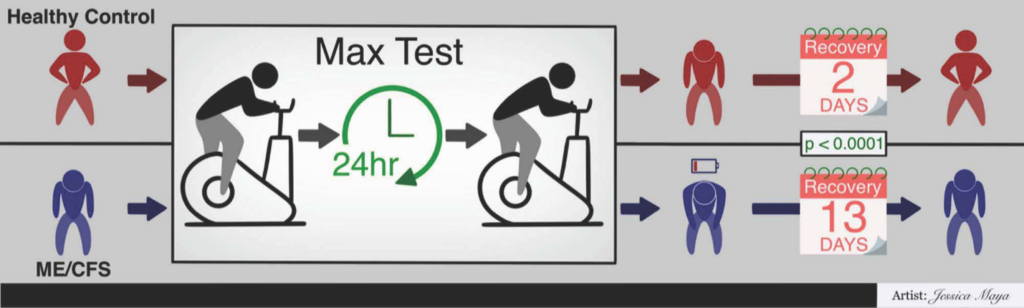
The article provides robust documentation of impaired recovery in people with ME/CFS following exertion (i.e., post-exertional malaise), something that is not seen in the control population. The study also recruited sedentary controls and used sex, age, and fitness-matched pairs to validate that fitness level does not predispose someone with ME/CFS to exertion intolerance. Keller et al. highlights that the autonomic nervous system is, at least partly, associated with ME/CFS due to the disrupted hemodynamic and ventilatory responses to exertion. Additionally, the worsening of CPET parameters over the two-day protocol led to increased clinical impairment status in ME/CFS whereas controls remain relatively the same.
Overall, the manuscript provides a detailed look at the physiological component of post-exertional malaise in ME/CFS. The paper is open access. Individuals interested in the topic should check out the full publication for more information.
 Center for Enervating NeuroImmune Disease
Center for Enervating NeuroImmune Disease