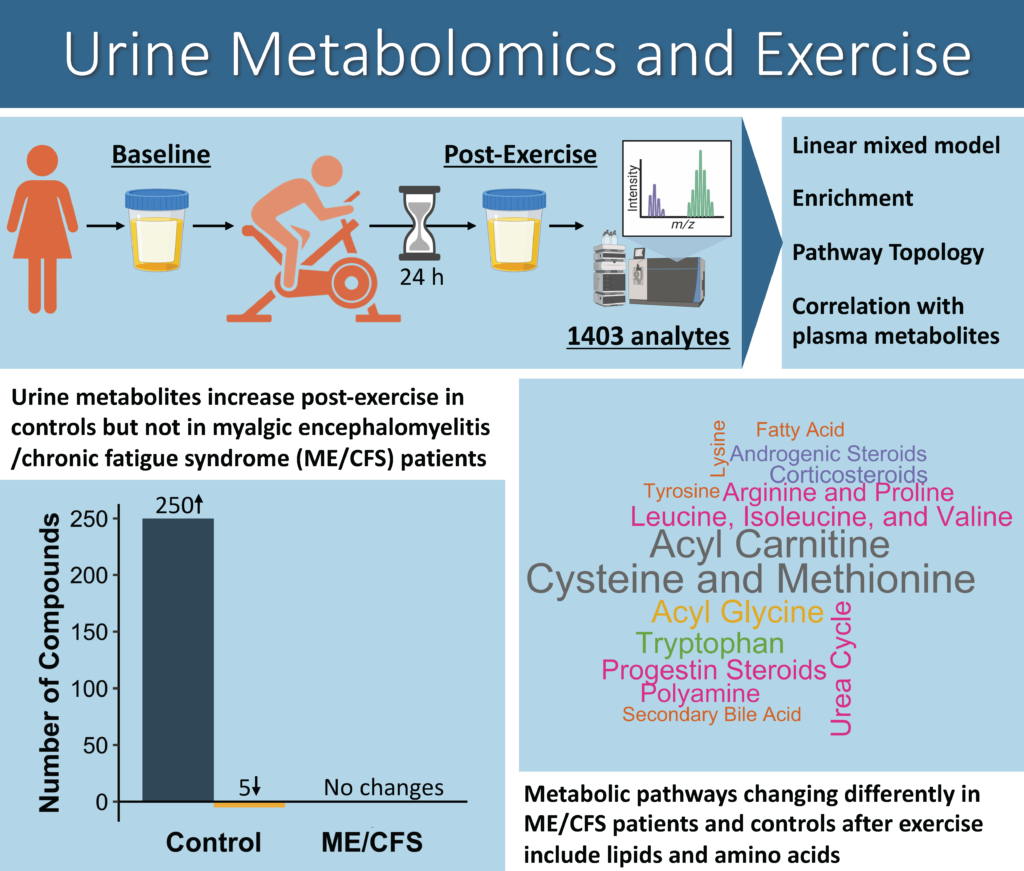
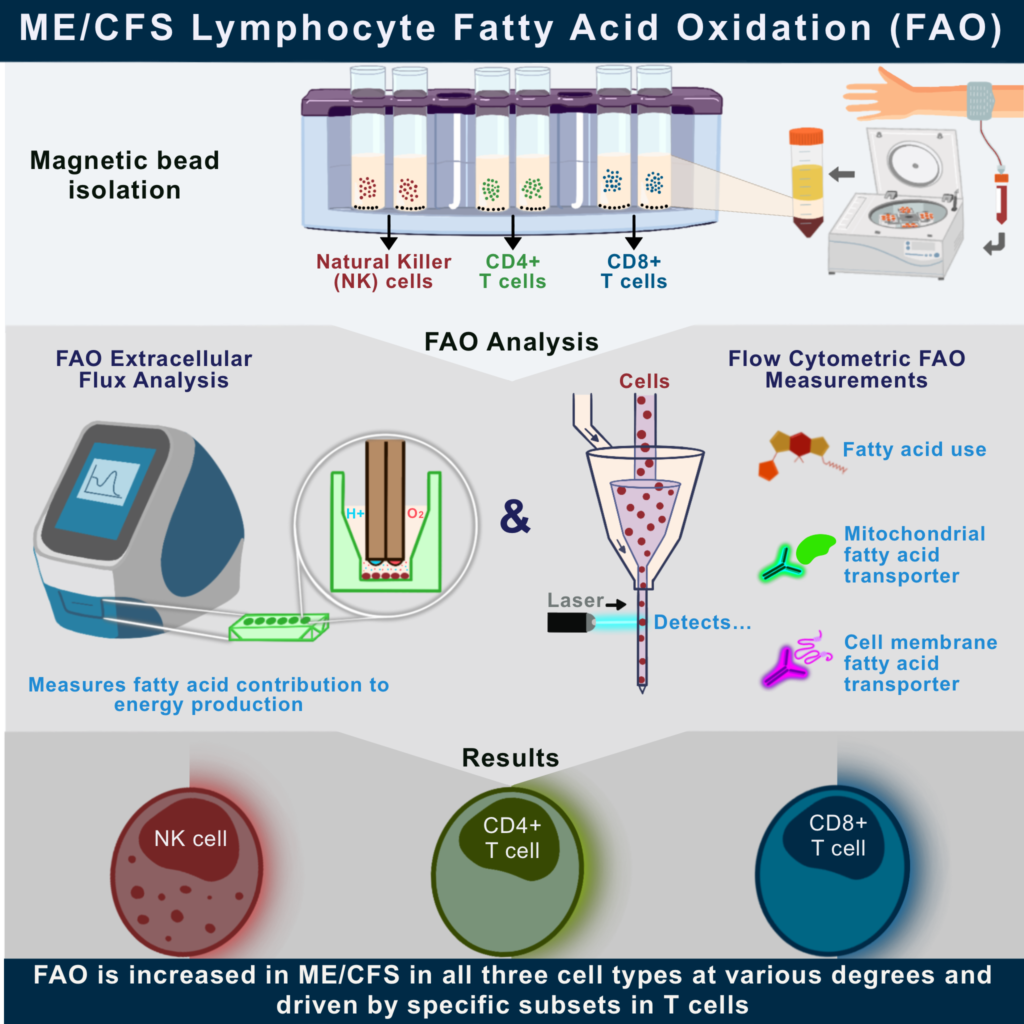
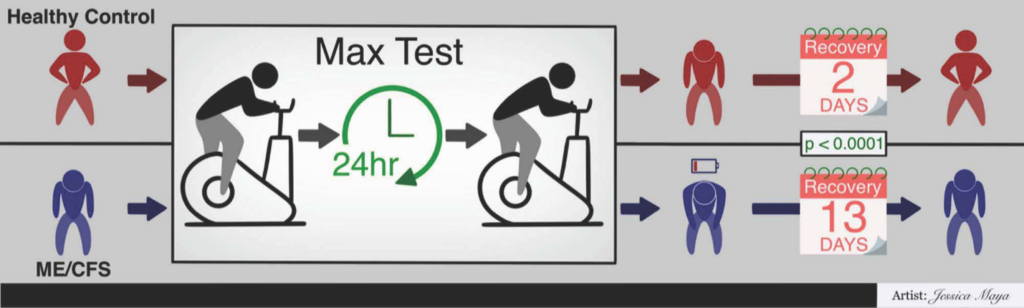
Cardiopulmonary exercise testing (CPET) was an integral part of our NIH-funded collaborative research center (CRC). The Cornell CRC used the CPET as a way to interrogate the hallmark symptom of ME/CFS—post-exertional malaise (PEM). CPET-associated samples are being analyzed to uncover the molecular basis of PEM. This molecular work gave us the opportunity to explore other aspects of PEM such as recovery following exertion.
Dr. Geoffrey Moore, M.D., Cornell CRC Clinical Core Co-director, led an effort to describe CPET recovery in ME/CFS. This work is now available in the journal Medicina under the title Recovery from Exercise in Persons with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). The paper documents a significant difference in recovery between sedentary controls (~2 days) and people with ME/CFS (~13 days). Moore et al. studied 84 people with ME/CFS and 60 controls using a self-reported symptom severity questionnaire. Both female and male participants from three different test sites across the United States were included in the study. The publication is open access so check it out for more information.
 Center for Enervating NeuroImmune Disease
Center for Enervating NeuroImmune Disease