Maureen Hanson spoke at the Demystifying Long COVID North American Conference, sponsored by Academic Medical Education organization, and her talk comparing ME/CFS with long COVID is now available online.
Category: Outreach
Center investigators present at International ME/CFS & Long COVID Conference
Center investigators presented at the 1st International Conference on Clinical and Scientific Advances in ME/CFS and Long COVID. Details regarding the agenda and event can be found on the event website. The two-day hybrid event was recorded. Both days of the conference are available below.
Day 1 (April 3, 2024): Dr. Susan Levine presented “Brief History of ‘Pandemics’ Related to ME/CFS Punta Gorda Florida and Royal Free Hospital in London. Origin of Current Case Definition.” Her talk starts at 27:00. Dr. Levine also took part in a panel discussion and “Conversation: The Role of Physician as Advocate, Lessons Learned from 30 Years of Clinical Practice” that occurred later in the day.
Day 2 (April 4, 2024): Dr. Maureen Hanson presented “Circulating Signals of ME/CFS.” Her talk starts at 6:07:00.
Center participates in the NIH ME/CFS Research Roadmap series
The working group of the National Advisory Neurological Disorders and Stroke Council, part of the National Institutes of Health, recommended the development of research priorities for ME/CFS to help move the field towards translational research and clinical trials. The result led to creation of the ME/CFS Research Roadmap Working Group. The Group, co-led by Drs. Lucinda Bateman and Maureen Hanson, produced a series of eight webinars. A complete description of the webinar series can be found on the ME/CFS Research Roadmap website.
Our Center’s mission is to promote research to identify its cause(s), biomarkers, and pathophysiology in order to lead to prevention and effective treatments. With this focus, several Center investigators presented data to support the development of research priorities for ME/CFS. The talks from our Center are included below.
Maureen Hanson presented “ Immune cell-type approaches to identify mechanisms of ME/CFS” during the Immune System webinar. Her talk starts at 1:38:40.
Jessica Maya presented “Investigations and Consequences of Altered Metabolism in ME/CFS Immune Cells” during the Metabolism webinar. Her talk starts at 58:32.
Maureen Hanson presented “Chronic infection in ME/CFS: non-Herpes viruses” during the Chronic Infections webinar. Her talk starts at 1:01:25.
Ludovic Giloteaux presented “Extracellular vesicles” during the Physiology webinar. His talk starts at 3:15:10.
The NIH ME/CFS Center presents lectures at “Advancing ME/CFS Research: Identifying Targets for Intervention and Learning from Long COVID”

NIH held a hybrid online conference on the NIH campus on December 11-12, 2023. Center personnel attending in person were Claire McNally, Annie Gardella, David Iu, Jessica Maya, Tien Luyen (“Louis”) Vu, Katherine Glass, Arnaud Germain, Ludovic Giloteaux, Dawei Li, Andrew Grimson, and Maureen Hanson, as well as collaborator Nicholas Hampilos from Weill Cornell Medicine.
Both days of the conference are available online.
Jessica Maya’s presentation “Investigating T cell populations for immune cell dysfunction in ME/CFS” starts at 3:50:10.
Maureen Hanson’s presentation on “ME/CFS and long COVID: similarities and differences” starts at 1:03:00.
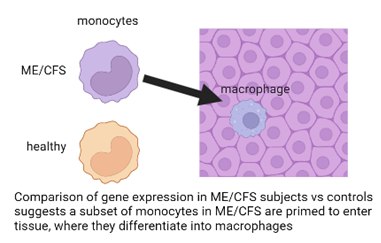
Andrew Grimson’s presentation on “Monocyte dysregulation in ME/CFS” starts at 1:37:35.
Nicholas Hampilos’ presentation on “Effect of Physical Exertion on CNS Oxidative Stress and Metabolism in ME/CFS” starts at 2:56:53.
Center trainees participate in the Symposium For Promoting The Advancement Of Research Knowledge In ME/CFS (SPARK ME)

NIH sponsored an early career researchers workshop on December 10, 2023 attended by Cornell graduate students Claire McNally Annie Gardella, and David Iu, postdoctoral associates Jessica Maya and Tien Luyen (“Louis”) Vu, and Research Associates Katherine Glass, Arnaud Germain and Ludovic Giloteaux. Drs. Glass and Maya helped organize the meeting.
Verbal presentations:
David Iu: Epigenetic Reprogramming of CD8+ T cell Populations Drives Exhaustion in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)
Tien Luyen Vu: Single-cell transcriptomics of ME/CFS circulating immune system before and after symptom provocation
Poster presentations:
Anne Gardella: Cell-free RNA signatures of myalgic encephalomyelitis/ chronic fatigue syndrome
Ludovic Giloteaux: Extracellular vesicle protein cargo in ME/CFS cases and controls following maximal exercise
Arnaud Germain: Proteomic adjustments following induction of post-exertional malaise
Claire McNally: Investigating the role of iNOS in endothelial dysfunction in ME/CFS
ME/CFS lecture presented at the Viral Infections and Inflammation Workshop in Rockville, Maryland September 6-7, 2023
In an NIH-sponsored workshop featuring studies of long COVID, acute COVID19, HIV/AIDS, viral encephalitis, Epstein-Barr Virus, Ebola virus, Endogenous retroviruses, Maureen Hanson presented a talk entitled “Viruses, Immune Dysfunction, and ME/CFS”
All talks can be accessed through the following website after free registration here or on the Academic Medical Education’s YouTube channel. Maureen Hanson’s talk is included below.
Urine metabolomics shows divergent response to exercise between people with ME/CFS and sedentary controls
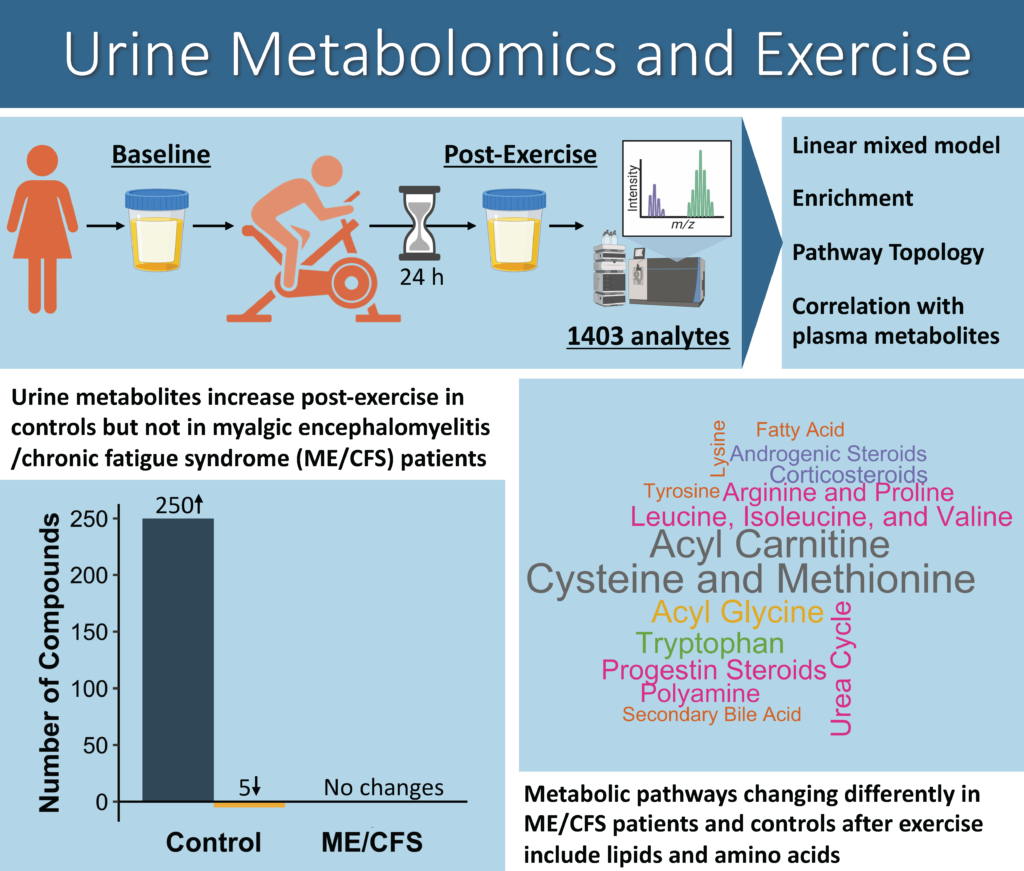
We have a new study published today that compares metabolite levels in urine of ME/CFS patients and sedentary controls before and after cardiopulmonary exercise testing (CPET).
Katie Glass is lead author of Urine Metabolomics Exposes Anomalous Recovery after Maximal Exertion in Female ME/CFS Patients. The study is available online in the International Journal of Molecular Sciences and full text is open access.

As shown in the graphical abstract above and explained in the video abstract below, we found a large number of metabolites at increased concentrations in the urine of controls 24 hours after CPET compared to baseline. However, we did not find significant changes in levels of any metabolites in the urine of ME/CFS patients after CPET.
When we looked at which metabolites were changing differently in ME/CFS patients and controls after exercise, we found the most compounds in the amino acid and lipid metabolic superpathways.
Overall, our data suggests that the metabolisms of sedentary controls undergo major changes that allow them to recover from exertion, while ME/CFS patients fail to make similar adaptive responses. This dysfunctional metabolic excretion could be contributing to exercise intolerance in ME/CFS patients.
Check out the paper to see many more results, including individual compounds that are significantly different between patients and controls and altered correlations between urine and plasma metabolites.
IACFS/ME’s October 2022 Journal Club features Arnaud Germain
Arnaud Germain, Center investigator, was featured at IACFS/ME’s October 2022 Journal Club. Germain presented and discussed his longitudinal ME/CFS metabolomics publication.
Head over to YouTube to watch the video using this link.
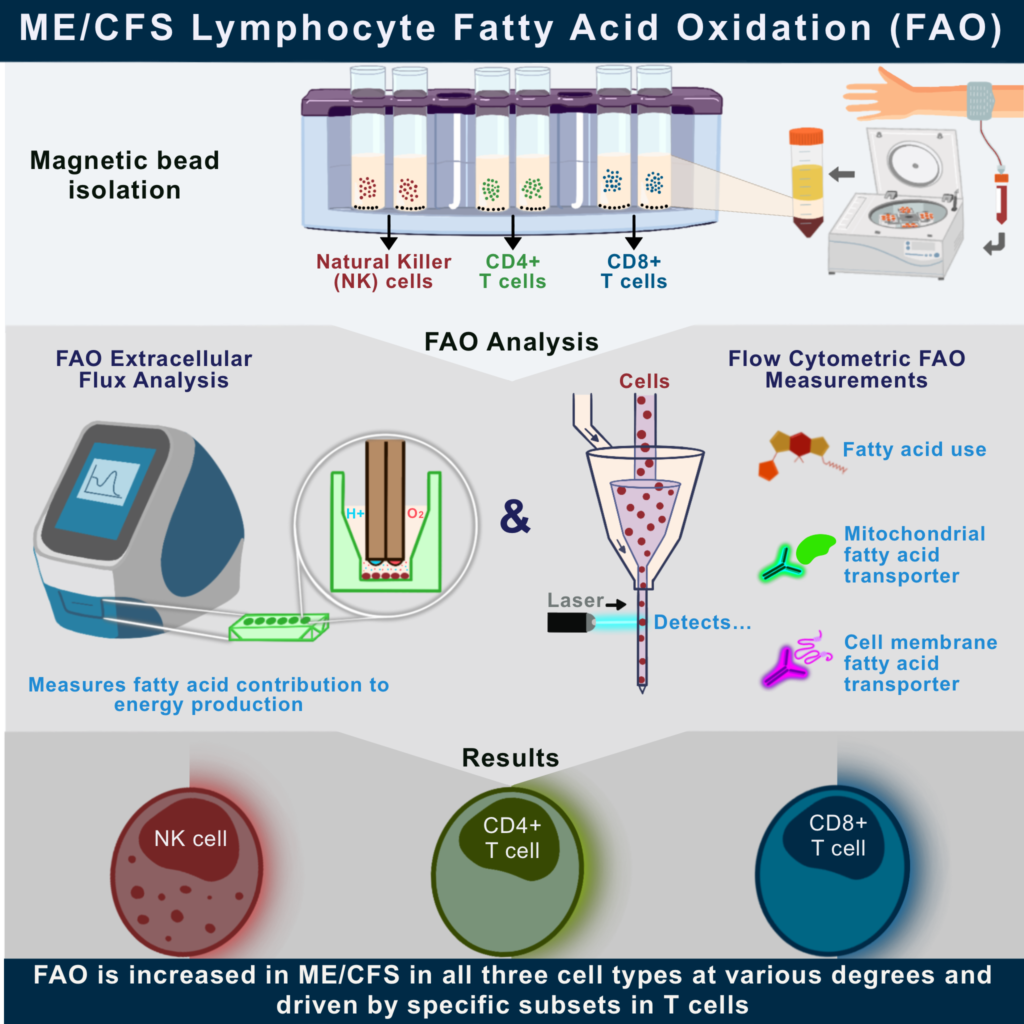
Fatty Acid Oxidation in ME/CFS Immune Cell Populations
A new publication from the Center on fatty acid oxidation in immune cells has appeared today. Jessica Maya is the lead author of Altered Fatty Acid Oxidation in Lymphocyte Populations of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome published in the International Journal of Molecular Sciences.
As discussed in the paper, there is more evidence for abnormal immunometabolism in ME/CFS. Maya utilized her expertise in flow cytometry and Seahorse flux analysis to demonstrate this dysfunction. She isolated natural killer (NK), helper T (CD4), and cytotoxic T (CD8) cell populations from both healthy donors and people with ME/CFS. These immune cell populations were studied in their circulating state and after stimulation. The stimulation process aims to mimic an immune response. Maya’s findings showed that all three of the cell types have an increased use of fats to power their activities when compared to healthy donors. Her results show that ME/CFS immune cells have a greater reliance on fats for energy when they are stimulated. Overall, these findings support the presence of an altered metabolic state in certain immune cells in individuals with ME/CFS.
Maya outlines these findings in her graphical and video abstracts inserted below.
A commentary by Andrew Grimson about the single-cell RNA-seq preprint from his lab
 Center for Enervating NeuroImmune Disease
Center for Enervating NeuroImmune Disease