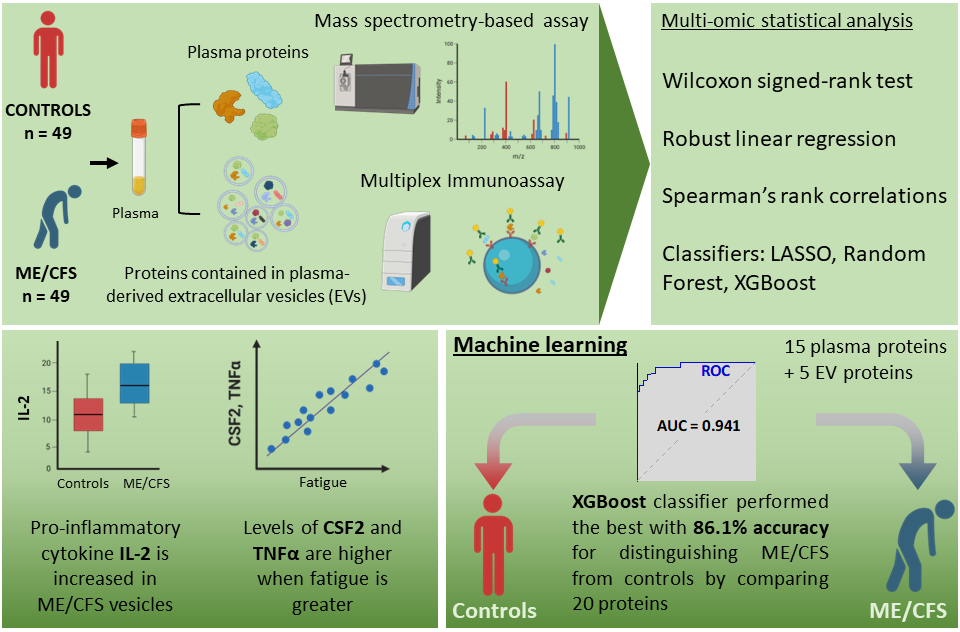
Exertion intolerance and post-exertional malaise are defining features of ME/CFS. Work from our Center aims to uncover the molecular disruption that occurs during and around these features. Thus, through the lens of extracellular vesicles (EVs), a publication from co-lead authors Ludovic Giloteaux and Katherine Glass provides novel insights into these topics.
The study involved the isolation of EVs, membrane-bound non-replicating particles released from cells, from the plasma of 18 ME/CFS and 17 healthy control female participants. The cargo of EVs are relevant in that they provide signals that could uncover elements of dysfunction in ME/CFS. Specifically, protein cargo from EVs were examined in this publication before and after a cardiopulmonary exercise test (i.e., around the induction of PEM).
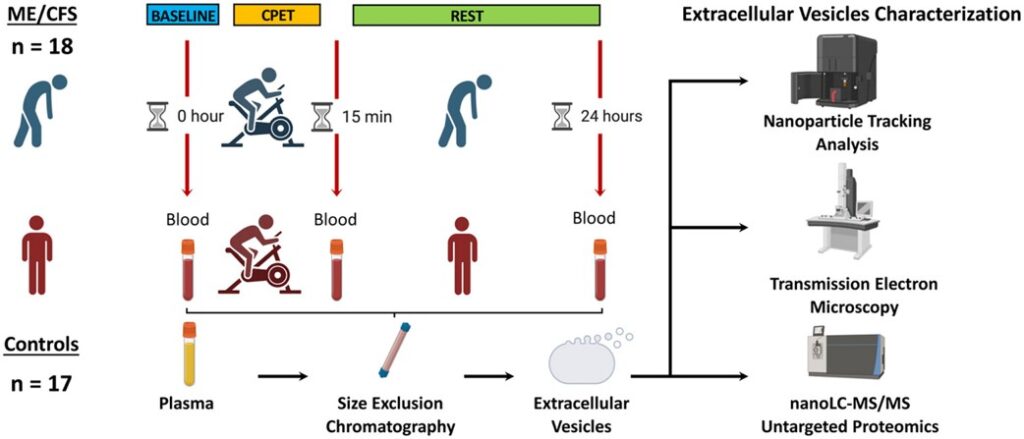
The EV proteome in response to exercise was clearly different in ME/CFS when compared to sedentary controls. These divergent responses were associated with different molecular pathways. Uncovered protein differences between the cohorts also point to contrasting tissues and cell types. Additionally, there were several proteins associated with a worsening of muscle pain (e.g., THBS1 and TPM4), PEM (e.g., NEXN), and fatigue (e.g., CLU) post exercise in ME/CFS. Although it is difficult to directly associate changes with the EV proteome to distinct cell types, this publication brings forth avenues to interrogate further.
If you are interested in more information regarding this publication, the entire paper is freely available in the Journal of Extracellular Vesicles.
 Center for Enervating NeuroImmune Disease
Center for Enervating NeuroImmune Disease